| VINYLIDENE CHLORIDE | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 75-35-4 |
|
| EINECS NO. | 200-864-0 | |
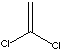
| FORMULA | H2C=CCl2 | |
| MOL WT. | 96.94 | |
|
H.S. CODE |
2903.19 | |
|
TOXICITY |
Oral ra LD50: 200 mg/kg | |
| SYNONYMS | 1,1-dichloroethylene; 1,1-Dichloroethene; DCE; | |
| asym-dichloroethylene; 1,1-DCE; vinylidene chloride(II); sconatex; vinylidene dichloride; ethylidene dichloride; Vinylidene Chloride Monomer; Chlorure de vinylidene; VDC; | ||
| SMILES | dehydrochlorination of 1,1,2-tricholoroethane | |
|
CLASSIFICATION |
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE | volatile colourless liquid | |
|
MELTING POINT |
super cold | |
| BOILING POINT | 32 C | |
| SPECIFIC GRAVITY | 1.213 | |
|
SOLUBILITY IN WATER |
insoluble | |
| pH | ||
| VAPOR DENSITY | 1.2 | |
| AUTOIGNITION |
570 C | |
|
REFRACTIVE INDEX |
1.425 | |
| NFPA RATINGS | Health: 2; Flammability: 4; Reactivity: 2 | |
| FLASH POINT |
5.6 C | |
| STABILITY | Stable under ordinary conditions | |
|
APPLICATIONS |
||
|
Vinyl: the univalent chemical radical H2C=CHCl-, derived from ethylene. It is a
highly reactive, easily polymerizing, and low cost monomer used as basic
materials for one of largest-selling plastic. In addition to its application of
polymerization to make plastics with huge amount, vinly- is an functional group
involved in cycloaddition, addition reactions, and carbon skeleton
expansion reactions including Suzuki reaction, Heck reaction.
This radical is useful in biomolecules chemistry such
as protein sequencing and enzyme inhibitors. Some vinyl compounds impart characteristic
flavors.
Vinylidene Chloride is used as a comonomer intermediate in the production of polymers with vinyl chloride, acrylonitrile, acrylates. It can be used to make food packing plastic wrap and flame retardant fabrics. It is used as an intermediate to produce adhesives, lacquer resins and refrigerant. |
||
| SALES SPECIFICATION | ||
|
APPEARANCE |
clear liquid | |
|
ASSAY |
99.0% min | |
|
COLOR, APHA |
10 max | |
|
ACIDITY |
0.01% max | |
|
WATER |
0.5% max | |
| TRANSPORTATION | ||
| PACKING | 240kgs in drum | |
| HAZARD CLASS | 3 (Packing group: I) | |
| UN NO. | 1303 | |
| REMARKS | ||
| Inhibited with monomethyl ether hydroquinone | ||